Direct naar Forum

|
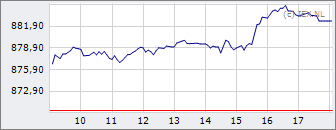
AEX
918,72
-5,89
-0,64%
14 jun
|
|
|
|

|
Germany40^ |
18.004,30
|
-1,43%
|
|

|
BEL 20 |
3.833,37
|
-0,92%
|
|

|
EURO50 |
4.840,53
|
0,00%
|
|

|
US30^ |
38.584,80
|
0,00%
|
|

|
Nasd100^ |
19.665,00
|
0,00%
|
|

|
US500^ |
5.432,55
|
0,00%
|
|

|
Japan225^ |
38.512,90
|
0,00%
|
|

|
Gold spot |
2.332,68
|
0,00%
|
|

|
EUR/USD |
1,0706
|
0,00%
|
|

|
WTI |
78,03
|
0,00%
|
|
#/^ Index indications calculated real time, zie
disclaimer
-
Premium


-
Premium


-
Premium


-
Premium


-
Premium

